Highlighting the New Advisory Committee on Immunization Practices (ACIP) Recommendation for 2017-2018 Influenza Season - (ACOP)
By, Carl Backes, DO, FACOP
Professor Pediatrics – OUHCOM
President, Kiddie West Pediatrics
President, ACOP
Here we go again. The hospitals are full again. It’s the flu season!
So why are we once again trying to get our patients vaccinated? Recently the AMA Wire discussed 6 reasons patients avoid the flu vaccination.
1 These reasons and responses are important to discuss with our patients:
- I am healthy, so I don’t need a flu vaccine.
- The flu vaccine isn’t safe and can give me the flu.
- If I get the flu, I will recover quickly.
- I’ll wait until the flu hits my area.
- I hate getting injections.
- I was vaccinated last year.
Recently, the conference highlights of “Influences and beliefs on vaccine hesitancy remain complex,” were published.
2 Dr. Frew of Emory University stated less than 1% of parents selectively or completely refuses all vaccines but an estimated 13% to 22% of parents intentionally delay vaccines. Her study categorized parents as non-hesitant acceptors of vaccines, hesitant acceptors, delayers or refusers. Surveys of 2,603 parents in 2012 and 2,518 parents in 2014 revealed that parents overwhelmingly cite
their health care provider as their most trusted source of information on vaccines including 99% of acceptors and 71% of refusers. Among hesitant acceptors 49% of parents in 2012 and 48% of parents in 2014 said their doctors
positively influenced their vaccination decision.
Judith Mendel, MPH of United States Department of Health and Human Services studied understanding vaccine-related confidence, how to overcome hesitancy over vaccines and what messages might work effectively. Recent concerns included:
3
- Persistent belief that autism is caused by vaccines
- Concern about vaccines made from weakened pathogens
- Belief that vaccines replace a function that the body is equipped to handle on its own
- Fears about short-term and long-term side effects
- Little tolerance for established reactions to vaccines
The message again was up to us, as the providers of health care! We must review published vaccine material and spend the time discussing all vaccine issues with parents.
Recent examples include:
- An article in JAMA Pediatrics (2017) regarding influenza infection and vaccinating during pregnancy and risk of autism spectrum disorder by O. Zerbo, PHD and colleague.4 They concluded “there was no association between maternal influenza infection anytime during pregnancy and increased ASD risk. There was suggestion of increased ASD risk among children whose mothers received an influenza vaccination in their first trimester, but the association was not statistically significant after adjusting for multiple comparisons, indicating that the finding could be due to chance. These findings do not call for changes in vaccine policy or practice, but do suggest the need for additional studies on maternal influenza vaccination and autism.”
- K. K. Bryant, M.D. reported on swine variant influenza virus infections, “In early August (2016) public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs. Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenza like illness, physicians and other frontline providers need to maintain an index of suspicion. From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who are at high risk for complications of influenza. Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is the key.5
- And finally, an article in Science Daily by Q. Sheikh and colleagues, “Scientists have designed a new generation of universal flu vaccines to protect against future global pandemics that could kill millions. Researchers have devised two universal vaccines; a USA-specific vaccine with coverage of 95% of known US influenza strains; and a universal vaccine with coverage of 88% of known flu strains globally.” 6
What do we face in 2017-2018?
(Weekly US Influenza Surveillance Report)
- As of May 5, 2018, 165 pediatric flu deaths, a cumulative rate of 106.5 laboratory confirmed influenza associated hospitalizations per 100,000 population was reported and 1.5% of outpatient visits for influenza-like illness.
- Most of the tested viruses are covered by the 2017 flu vaccine – approximately 96% of all antigenically characterized influenza A viruses and 94.9% of influenza viruses.
- Nationally, 31,488 cases A(H3) – predominant.
First and foremost, how about the 2017-2018 prevention and control of Influenza?
Introduction 7-10
Influenza viruses typically circulate widely in the United States annually, from late fall through early spring. Although most persons who become infected with influenza viruses will recover without sequelae, influenza can cause serious illness and death, particularly among older adults, very young children, pregnant women, and those with chronic medical conditions. During 31 seasons from the 1976–77 through the 2006–07 season, estimated influenza-associated deaths ranged from approximately 3,300 to 49,000 annually.11 Annual influenza vaccination is the primary means of preventing influenza and its complications. A variety of different types of influenza vaccine are available.
Abbreviation conventions for the different types of vaccine have evolved over time (Table 1). Routine annual influenza vaccination for all persons aged ≥6 months that do not have contraindications has been recommended by CDC and CDC’s Advisory Committee on Immunization Practices (ACIP) since 2010.
Table 1.
Influezna vaccines - United States, 2016-17 influenza season |
(Click to enlarge Table 1)
 |
Methods
ACIP provides annual recommendations for the prevention and control of influenza. Updates to the recommendations described in this document are of 3 types 1) the vaccine viruses included in the 2017-2018 seasonal influenza vaccines, 2) new vaccine licensures and approvals, and 3) an interim recommendation that live attenuated influenza vaccine (LAIV4) not be used during the 2017-2018 season.
Recommendations for vaccine viruses to be included in North Hemisphere influenza vaccines are made by the World Health Organization (WHO), which organizes a consultation, generally in February of each year, to make recommendations for vaccine composite.
12
(Background and Epidemiology)
Biology of Influenza
Influenza A and B are the two types of influenza viruses that cause epidemic human disease. Influenza A and B viruses are further separated into subtypes (for A viruses) and lineages (for B viruses) based on antigenic differences. Influenza A viruses are categorized into subtypes based on the characterization of two surface antigens: hemagglutinin (HA) and neuraminidase (NA). Influenza A (H1N1) viruses, influenza A (H3N2) viruses, and influenza B viruses co-circulate globally. Antibody against one influenza virus type or subtype confers limited or no protection against another type or subtype. Frequent emergence of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and necessitates consideration for adjustment of vaccine viruses each season. Larger genetic changes, or antigenic shifts, occur among influenza A viruses, less frequently than antigenic drift events. The HA gene is believed to have evolved from the avian-origin 1918 pandemic influenza A (H1N1) virus and is thought to have entered human and swine populations at about the same time. Influenza B viruses are separated into two distinct genetic lineages (Yamagata and Victoria) but are not categorized into subtypes. Influenza B viruses undergo antigenic drift less rapidly than influenza A viruses.13
Burden of Influenza Illness
Although precise timing of the onset, peak, and end of influenza activity varies from one season to the next, annual epidemics of seasonal influenza typically occur in the United States between October and April. Increases in health care provider visits for acute febrile respiratory illness occur annually, coinciding with periods of increased influenza activity, making influenza-like illness (ILI) surveillance systems valuable in understanding the seasonal and geographic occurrence of influenza each year.
Persons of all age groups are susceptible to influenza. Data from the Influenza Incidence Surveillance Project (IISP) covering the 2009-10 through 2012-13 seasons revealed the highest rates of outpatient visits for influenza-positive ILI occurred among children aged 2 through 17 years. Complications, hospitalizations, and deaths from seasonal influenza are typically greatest among persons aged ≥65 years, children aged <5 years (and particularly those aged <2 years), and persons of any age who have medical conditions that confer increased risk for complications from influenza. In typical winter influenza seasons, increases in deaths and hospitalizations are observed during periods when influenza viruses are circulating.
- Children
Influenza is an important cause of outpatient medical visits and hospitalizations among young children. Estimated rates of influenza-associated hospitalization generally are substantially higher among infants and children aged <5 years than among older children. In the United States, death associated with LCI among children aged <18 years has been a nationally reportable condition since October 2004. Since reporting began, the annual number of influenza-associated pediatric deaths during regular influenza seasons has ranged from 37 to 171 deaths per season. A larger number of deaths were reported during the 2009 pandemic, for which 358 pediatric deaths were reported to CDC from April 15, 2009 through October 2, 2010.14 As of May 8, 2018 – 165 pediatric deaths.
- Pregnant Women and Neonates
Pregnant women are vulnerable to severe symptoms and illness attributable to influenza. Physiologic changes associated with pregnancy, such as altered respiratory mechanics and changes in cell mediated immunity, might contribute to enhanced susceptibility. In a case-cohort study of 1,873 pregnant women conducted over the 2010-11 and 2011-12 seasons, among 292 women with acute respiratory illnesses, those with influenza reported greater symptom severity than those with non-influenza acute respiratory illness. Pregnant women who were treated with antivirals >4 days after symptom onset were more likely to be admitted to an intensive care unit (57% versus 9%; relative risk [RR]: 6.0; 95% Cl = 3.5 – 10.6) than those treated within 2 days after symptom onset. A cohort study conducted among 221 hospitals in the United Kingdom observed an increased risk for perinatal death, stillbirth, and preterm birth among women admitted with confirmed 2009 (H1N1) infection. Notably, influenza symptoms often include fever, which during pregnancy might be associated with neural tube defects and other adverse outcomes. A meta-analysis of 22 observational studies of congenital anomalies, including neural tube defects, hydrocephaly, heart and aortic valve defects, digestive system defects, cleft lip, and limb reduction defects. Additional studies are needed to further elucidate the association between influenza and congenital anomalies and other birth outcomes.
- Persons with Increased Risk for Severe Influenza Illness and Complications
In the first U.S. recommendations for annual influenza vaccination, published by the Surgeon General in 1960, persons with “chronic debilitating diseases” (particularly cardiovascular disease, pulmonary disease, and diabetes) were cited as being among the groups contributing most to the excess deaths observed during the 1957 influenza pandemic.
Influenza Vaccine Immunogenicity and Effectiveness
Estimates of vaccine efficacy (i.e., prevention of illness among vaccinated persons enrolled in controlled clinical trials) and vaccine effectiveness (i.e., prevention of illness in vaccinated populations) of influenza vaccines depend on many factors, including the age and immunocompetence of the vaccine recipient, the degree of similarity between the viruses in the vaccine and those in circulation, study design, diagnostic testing measures, and the outcome being measured. A sample from the National Inpatient Sample noted a decrease in the number of hospitalizations associated with P&I of 295,000 and a decrease of 13,600 P&I-associated inpatient deaths for October 2008 through December 2011, compared with what would have been expected based on previous rates.
Immune Response Following Vaccination
Humoral and cell-mediated responses to influenza vaccination among children and adults have been studied. Serum antibodies are correlates of vaccine-induced protection for inactivated influenza vaccines (IIVs). Increased levels of antibody induced by vaccination decrease the risk for illness caused by strains that are antigenically like those strains of the same type or subtype included in the vaccine.
Influenza Vaccine Effectiveness and Match Between Vaccine and Circulating Viruses
Selection of viruses is based on consideration of global influenza surveillance data, from which decisions are made regarding the viruses most likely to circulate during the upcoming season. During some seasons, because of antigenic drift among influenza A viruses or change in predominant lineage among B viruses, circulating viruses might differ from those included in the vaccine.
Duration of Immunity
Evidence from some clinical trials indicates that protection against viruses that are antigenically like those contained in the vaccine extends at least for 6–8 months, particularly in nonelderly populations.
Immunogenicity, Efficacy, and Effectiveness of IIV
IIVs are administered by intramuscular or intradermal injection and contain nonreplicating virus. Immunogenicity, effectiveness, and efficacy have been evaluated in children and adults, although fewer data from randomized studies are available for certain age groups. In general, prelicensure studies of immunogenicity of the currently licensed IIV4s compared with corresponding IIV3 products (e.g., Fluzone Quadrivalent versus Fluzone, Fluarix Quadrivalent versus Fluarix, and Flulaval Quadrivalent versus Flulaval) demonstrated superior immunogenicity for IIV4 for the added influenza B virus without interfering with immune responses to the remaining three vaccine viruses.
Children: Children aged ≥6 months typically develop protective levels of antibodies against specific influenza virus strains after receiving the recommended number of doses of seasonal IIV. Immunogenicity studies using the A(H1N1) pdm09 monovalent vaccine indicated that 80%–95% of vaccinated children developed protective antibody levels to the 2009 A(H1N1) influenza virus after 2 doses; response after 1 dose was 50% for children aged 6–35 months and 75% for those aged 3–9 years. Estimates of the efficacy of IIV among children aged ≥6 months vary by season and study design. In a randomized, double-blind, placebo-controlled trial conducted during two influenza seasons among 786 children aged 6–24 months, estimated efficacy was 66% (95% CI = 34–82) against culture-confirmed influenza illness during 1999–2000. However, vaccination did not reduce culture-confirmed influenza illness significantly during 2000–2001, when influenza attack rates were lower. Receipt of IIV was associated with a reduction in acute otitis media in some studies but not in others.
Pregnant Women and Neonates
IIV induces protective levels of antibody in pregnant women. Passive transfer of anti- influenza antibodies from vaccinated women to neonates has been documented. In a randomized controlled trial conducted in Bangladesh, vaccination of pregnant women during the third trimester resulted in a 36% reduction in respiratory illness with fever among these women, as compared with women who received pneumococcal polysaccharide vaccine.
Persons with Chronic Medical Conditions
In a nonrandomized controlled trial during the 1992–93 season involving 137 children who had moderate to severe asthma, vaccine efficacy against laboratory- confirmed influenza A(H3N2) infection was 54% among children aged 2 through 6 years and 78% among children aged ≥7 through 14 years.
Immunocompromised Persons
In a comparative study of 41 children and young adults aged 3–21 years with cancer or HIV infection, high-dose IIV3 was no more immunogenic than standard-dose IIV3 among the HIV-infected recipients.
Highlights and Specific Guidelines
2017-2018 Season Highlights
- Annual universal influenza immunization is indicated with either a trivalent or quadrivalent (no preference) inactivated vaccine.
- There are many different flu viruses and they are constantly changing. The composition of U.S. flu vaccines is reviewed annually and updated as needed to match circulating flu viruses. Flu vaccines protect against the three or four viruses (depending on vaccine) that research suggests will be most common. For 2017-2018, trivalent vaccines are recommended to contain the following (https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm):
- an A/Michigan/45/2015 (H1N1) pdm09-like virus (updated)
- an A/Hong Kong/4801/2014 (H3N2)-like virus
- a B/Brisbane/60/2008-like (B/Victoria lineage) virus
- In addition, the quadrivalent vaccine contains: B/Phuket/3073/2013-like (B/Yamagata lineage) virus.
- Quadrivalent live attenuated influenza vaccine (LAIV4) should not be used in any setting during the 2017–2018 influenza season in light of the evidence for poor effectiveness of LAIV4 in recent seasons, particularly against influenza A (H1N1) pdm09 viruses.
- All children with egg allergy can receive influenza vaccine with no additional precautions from those of routine vaccinations.
- All HCP should receive an annual influenza vaccine, a crucial step in preventing influenza and reducing health care–associated influenza infections. Because HCPs may care for or live with people at high risk of influenza-related complications, it is especially important for them to get vaccinated annually.
- Pediatricians should attempt to promptly identify children suspected of having influenza for rapid antiviral treatment, when indicated, to reduce morbidity and mortality.
The American Academy of Pediatrics (AAP) recommends annual seasonal influenza vaccination for everyone 6 months and older, including children and adolescents, during the 2017-2018 influenza season. Special effort should be made to vaccinate the people in the following groups:
- All children, including infants born preterm, aged 6 months and older (based on chronologic age) with conditions that increase the risk of complications from influenza (e.g., children with chronic medical conditions, such as asthma, diabetes mellitus, hemodynamically significant cardiac disease, immunosuppression, or neurologic and neurodevelopmental disorders).
- All household contacts and out of home care providers of children with high-risk conditions and those younger than 5 years, especially infants younger than 6 months.
- American Indian/Alaska Native children.
- All health care personnel (HCP).
- All child care providers and staff.
- All women who are pregnant, are considering pregnancy, are in the postpartum period, or are breastfeeding during the influenza season.
Key points relevant for the 2017-2018 Influenza season
- It is important that household contacts and out-of-home care providers of children younger than 5 years, especially infants younger than 6 months, and children of any age at high risk of complications from influenza (e.g., children with chronic medical conditions, such as asthma, diabetes mellitus, hemodynamically significant cardiac disease, immunosuppression, or neurologic and neurodevelopmental disorders) receive annual influenza vaccine. In the United States, more than two-thirds of children younger than 6 years and almost all children 6 years and older spend significant time in child care or school settings outside the home. Exposure to groups of children increases the risk of contracting infectious diseases. Children younger than 2 years are at increased risk of hospitalization and complications attributable to influenza. School-aged children bear a large influenza disease burden and have a significantly higher chance of seeking influenza-related medical care compared with healthy adults. Reducing influenza virus transmission (e.g., by using appropriate hand hygiene and respiratory hygiene/cough etiquette) among children who attend out-of- home child care or school has been shown to decrease the burden of childhood influenza and the transmission of influenza virus to household contacts and community members of all ages.
- Considering the evidence for poor effectiveness of quadrivalent live attenuated influenza vaccine (LAIV4) documented during the past 3 seasons, particularly against influenza A (H1N1) pdm09 viruses, LAIV4 should not be used in any setting during the 2017–2018 season.
- Vaccination remains the best available preventative measure against influenza. Vaccination is effective in reducing outpatient medical visits for illness caused by circulating influenza viruses by 50% to 75%.
- Both trivalent and quadrivalent IIVs are available in the United States for the 2017–2018 season. To vaccinate as many people as possible for this influenza season, neither inactivated vaccine formulation is preferred over the other.
- The number of seasonal influenza vaccine doses to be administered in the 2017–2018 influenza season depends on the child’s age at the time of the first administered dose and his or her vaccine history.
- Influenza vaccines are not licensed for administration to infants younger than 6 months.
- Children aged 9 years and older need only 1 dose.
- Children 6 months through 8 years of age:
- Need 2 doses if they have received fewer than 2 doses of any trivalent or quadrivalent influenza vaccine (IIV or LAIV) before July 1, 2016. The interval between the 2 doses should be at least 4 weeks.
- Require only 1 dose if they have previously received 2 or more total doses of any trivalent or quadrivalent influenza vaccine (IIV or LAIV) before July 1, 2016. The 2 previous doses do not need to have been received during the same influenza season or consecutive influenza seasons. Despite recent evidence for poor effectiveness of LAIV4, receipt of LAIV4 in the past is still expected to have primed a child’s immune system. There currently are no data that suggest otherwise. Therefore, children who received 2 or more doses of LAIV4 before July 1, 2016 may receive only 1 dose of IIV for the 2017–2018 season.
- Pregnant women can receive influenza vaccine safely at any time during pregnancy. Pregnant women are of special concern because they are at high risk of complications from influenza. Vaccination of pregnant women also provides protection for their infants, potentially for as long as 6 months through the transplacental transfer of antibodies.
- Providers may continue to offer vaccine until June 30th of each year, marking the end of the influenza season, because influenza is so unpredictable. Protective immune responses generally persist in children throughout the influenza season. Although peak influenza activity in the United States tends to occur in January through March, influenza activity can occur in early fall (October) or in late spring (end of May) and may have more than 1 disease peak.
- HCP should act as role models for both their patients and colleagues by receiving influenza vaccination annually and by letting others know that they have received vaccine, highlighting the safety and effectiveness of annual influenza vaccination. Influenza vaccination programs for HCP benefit the health of employees, their patients, and members of the community. Mandatory influenza immunization for all HCP is ethical, just, and necessary to improve patient safety. 2 Employees of health care institutions are obligated to act in the best interests of the health of their patients and to honor the requirement of causing no harm.
- Antiviral medications also are important in the control of influenza but are not a substitute for influenza vaccination. The neuraminidase inhibitors (NAIs) oral oseltamivir (Tamiflu; Roche Laboratories, Nutley, NJ) and inhaled zanamivir (Relenza; GlaxoSmithKline, Research Triangle Park, NC) are the only antiviral medications that are recommended for chemoprophylaxis or treatment of influenza in children during the 2016–2017 season. Intravenous zanamivir remains investigational but can be used in consultation with infectious diseases specialists, and it may also be obtained on a compassionate-use basis for seriously ill children, as currently supported by the US Food and Drug Administration (FDA) through the manufacturer, GlaxoSmithKline. This information is especially important for those who are immunocompromised or who cannot tolerate or absorb orally or enterically administered oseltamivir. Intravenous zanamivir is being studied in pediatric patients, but the manufacturer has not publicly released any information regarding any plans to file for licensure in adults or children.
Seasonal Influenza Vaccines
IIVs
For the 2017–2018 season, IIVs will be available for intramuscular injection in both trivalent (IIV3) and quadrivalent (IIV4) formulations. IIVs do not contain live virus. The intramuscular formulations can be used in children with and without chronic medical conditions. The most common adverse events after IIV3 administration are local injection site pain and tenderness. Fever occurs within 24 hours after immunization in approximately 10% to 35% of children younger than 2 years but rarely in older children and adults. Mild systemic symptoms, such as nausea, lethargy, headache, muscle aches, and chills, may occur after administration of IIV3. Intramuscular formulations of IIV4 are available from several manufacturers. Different formulations have different age indications, but there are brands licensed for use in children as young as 6 months of age. In children, the most common injection site adverse reactions were pain, redness, and swelling. The most common systemic adverse events were drowsiness, irritability, loss of appetite, fatigue, muscle aches, headache, arthralgia, and gastrointestinal tract symptoms. These events were reported with comparable frequency among participants receiving the licensed comparator IIV3. IIV4 is an acceptable vaccine for people 6 months or older when otherwise appropriate and may offer broader protection against circulating influenza B strains than IIV3. During the 2 influenza seasons spanning 2010–2012, there were increased reports of febrile seizures in the United States in young children who received IIV3 and the 13-valent pneumococcal conjugate vaccine (PCV [PCV13]) concomitantly. The concomitant administration of IIV3, PCV, and DTaP was associated with the greatest relative risk. Data from the FDA’s Post Licensure Rapid Immunization Safety Monitoring (PRISM) program, the largest vaccine safety surveillance program in the United States, revealed that there was no significant increase in febrile seizures associated with concomitant administration of these 3 vaccines in children 6 to 59 months of age during the 2010–2011 season. Overall, the benefits of timely vaccination with same-day administration of IIV and PCV or DTaP outweigh the risk of febrile seizures. In children aged 4 through 17 years, injection site tenderness and erythema were the most common (≥10%) local reactions, and the most common (≥10%) systemic reactions included sleepiness/fatigue and irritability/ headache. A large body of scientific evidence shows that thimerosal-containing vaccines are not associated with an increased risk of autism spectrum disorders in children.
Influenza vaccine and egg allergy
Although most IIV vaccines are produced in eggs and contain measurable amounts of egg protein, recent data have shown that IIV administered in a single, age appropriate dose is well tolerated by recipients with a history of egg allergy of any severity. Recent literature has shown that egg allergy does not impart an increased risk of anaphylactic reaction to vaccination with IIV. A 2012 review of published data found no instances of anaphylaxis among 4172 egg allergic patients, 513 of whom had a history of severe egg allergy, after vaccination with influenza vaccine; some did have milder reactions. According to a Vaccine Safety Datalink study, the rate of anaphylaxis after IIV3 administration is about 1 per 1 000 000 doses (10 cases in almost 7.5 million doses given alone from 2009 to 2011). This rate is not different from those of other vaccines, including ones that do not contain egg. Although a waiting period of 30 minutes after vaccination for patients with egg allergy was previously recommended, this study also found that the onset of symptoms of anaphylaxis after receiving any vaccine began more than 30 minutes later in 21 of 29 cases. In addition, ccIIV4 is anticipated to be available for people 4 years and older during the 2016–2017 season.
Vaccine Storage and Administration
IIVs for intramuscular injection are shipped and stored at 2° to 8°C (36°–46°F); frozen vaccines should not be used. These vaccines are administered intramuscularly into the anterolateral thigh of infants and young children and into the deltoid muscle of older children and adults. The volume of vaccine is age dependent; infants and toddlers 6 months through 35 months of age should receive a dose of 0.25 mL, and all persons 3 years (36 months) and older should receive a dose of 0.5 mL. A 0.5-mL unit dose of any IIV should not be split into 2 separate 0.25-mL doses because of safety concerns for lack of sterility, variance with the package insert, and potential compliance difficulties with vaccine excise taxes.
Current Recommendations - 2018 Forward
(as of February 21, 2018)
The U.S. Centers for Disease Control and Prevention (CDC) Vaccine Advisory Group (ACIP) voted to include FluMist in the vaccine line up for the 2018 – 2019 flu season, returning to the US market after a two-season hiatus.
- Astra Zeneca has swapped out the previous 2009 H1N1 with a different type (A/Slovenia). Validated study confirms improved H1N1 – LAIV strain.16
Seasonal influenza vaccination with IIV is recommended for all children 6 months and older. LAIV can be used 2018-2019. Children and adolescents with certain underlying medical conditions have an elevated risk of complications from influenza, which include the following:
- Asthma or other chronic pulmonary diseases, including cystic fibrosis;
- Hemodynamically significant cardiac disease;
- Immunosuppressive disorders or therapy;
- HIV infection;
- Sickle cell anemia and other hemoglobinopathies;
- Diseases that necessitate long-term aspirin therapy, including juvenile idiopathic arthritis or Kawasaki disease;
- Chronic renal dysfunction;
- Chronic metabolic disease, including diabetes mellitus;
- Any condition that can compromise respiratory function or handling of secretions or that can increase the risk of aspiration, such as neurodevelopmental disorders, spinal cord injuries, seizure disorders, or neuromuscular abnormalities; and
- Pregnancy
Additional vaccination efforts should be made for the following groups to prevent transmission of influenza to those at risk, unless contraindicated:
- Household contacts and out of home care providers of children younger than 5 years and of at-risk children of all ages
- Any woman who is pregnant or considering pregnancy, is in the postpartum period, or is breastfeeding during the influenza season. Studies have shown that infants born to immunized women have better influenza-related health outcomes compared with infants of unimmunized women. However, according to Internet based panel surveys conducted by the CDC, only approximately 50% of pregnant women reported receiving an influenza vaccine during the 2014–2015 season, even though both pregnant women and their infants are at higher risk of complications. In addition, data from some studies suggest that influenza vaccination in pregnancy may decrease the risk of preterm birth and infants being small for gestational age. Breastfeeding also is recommended to protect against influenza viruses by activating innate antiviral mechanisms, specifically type 1 interferons. In addition, the breast milk of mothers vaccinated during the third trimester contains higher levels of influenza-specific immunoglobulin A. Greater exclusivity of breastfeeding in the first 6 months of life decreases the episodes of respiratory illness with fever in infants of vaccinated mothers. Pregnant women can receive influenza vaccine safely during any trimester.
- American Indian/Alaska Native children and adolescents
- HCP or health care volunteers. Despite the AAP recommendation for mandatory influenza immunization for all health care personnel, 2 many remain unvaccinated. With an increasing number of organizations mandating influenza vaccine, coverage among HCP increased to 77% in the 2014–2015 season. The optimal prevention of influenza in the health care setting depends on the vaccination of at least 90% of HCP, which is consistent with the national Healthy People 2020 target for annual influenza vaccination among HCP. However, overall vaccination rates for this group remain consistently below this goal. The AAP recently reaffirmed its support for a mandatory influenza vaccination policy for all HCP nationwide. Mandating influenza vaccine for all HCP is ethical, just, and necessary to improve patient safety, especially because HCP frequently encounter patients at high risk of influenza illness in their clinical settings. For the prevention and control of influenza, all HCP must continue to put the health and safety of patients first.
- Close contacts of immunosuppressed people.
Contraindications and Precautions
Minor illnesses, with or without fever, are not contraindications to the use of influenza vaccines, particularly among children with mild upper respiratory infection symptoms or allergic rhinitis. Children with moderate to severe febrile illness, based on the judgment of the clinician, should not be vaccinated with IIV until resolution of the illness. Infants younger than 6 months should also not be vaccinated with IIV. A previous severe allergic reaction (i.e., anaphylaxis involving cardiovascular changes, respiratory or gastrointestinal tract symptoms, or reactions that necessitate the use of epinephrine) to influenza vaccine, regardless of the component suspected of being responsible for the reaction, continues to be a contraindication to future receipt of the vaccine. The estimated risk of Guillain-Barre syndrome (GBS) is low, especially in children. As a precaution, people who are not at high risk of severe influenza and who are known to have experienced GBS within 6 weeks of influenza vaccination generally should not be vaccinated. However, the benefits of influenza vaccination might outweigh the risks for certain people who have a history of GBS and who also are at high risk of severe complications from influenza.
Use of Antiviral Medications
Oral oseltamivir remains the antiviral drug of choice for the management of influenza infections. Inhaled zanamivir is an equally acceptable alternative for patients who do not have chronic respiratory disease. However, it is more difficult to administer. Antiviral resistance to either drug can emerge, necessitating continuous population based assessment that is conducted by the CDC. If local or national influenza surveillance data indicate the emergence of an influenza strain with a known antiviral resistance profile, then empirical treatment can be directed toward that strain with an effective antiviral agent. During the 2015–2016 season, the great majority of influenza strains were susceptible to oseltamivir, zanamivir, and peramivir. In contrast, high levels of resistance to amantadine and rimantadine exist, so these drugs should not be used in the upcoming season unless resistance patterns change significantly. If the commercially manufactured oral suspension is not available, the capsule may be opened, and the contents mixed with simple syrup or Ora-Sweet SF (sugar-free) by retail pharmacies to a final concentration of 6 mg/mL. The greatest effect on outcome will occur if treatment can be initiated within 48 hours of illness onset but still should be considered if later during illness. In previous years, the use of double dose oseltamivir, particularly for those hospitalized with severe illness caused by influenza A (H1N1) pdm09, was believed to provide better outcomes compared with standard dosing.
| TABLE 2. |
Recommended Dosage and Schedule of Influenza Antiviral Medications for Treatment and Chemoprophylaxis for the 2017–2018 Influenza Season: United States
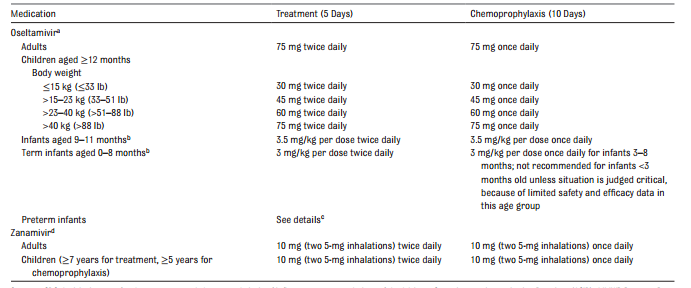
 |
The FDA has licensed oseltamivir for children as young as 2 weeks of age. Given preliminary pharmacokinetic data and limited safety data, the AAP believes that oseltamivir can be used to treat influenza in both term and preterm infants from birth because benefits of therapy are likely to outweigh the possible risks of treatment.
A review of controlled clinical trial data and ongoing surveillance failed to establish a link between this drug and neurologic or psychiatric events.
Antiviral treatment should be started as soon as possible after illness onset and should not be delayed while waiting for a definitive influenza test result, because early therapy provides the best outcomes. Positive results of rapid influenza tests are helpful, because they may reduce additional testing to identify the cause of the child’s influenza-like illness. These molecular assays or polymerase chain reaction (PCR) test confirmation are preferred in hospitalized patients.
Persons with suspected influenza who present with an uncomplicated febrile illness should be offered treatment with antiviral medications if they are at higher risk of influenza complications, particularly if he or she is in contact with other children who either is younger than 6 months or who have underlying medical conditions that predispose them to complications of influenza.
Sources
1)
O'Reilly, K. B. (2016, December 08). 6 reasons patients avoid flu vaccination. Retrieved January 04, 2017, from https://wire.ama-assn.org/
2)
Haelle, T. (2016, December 02). Influences and beliefs on vaccine hesitancy remain complex. Retrieved January 4, 2017, from http://www.mdedge.com/pediatricnews/article/119345/vaccines/influences-and- beliefs-vaccine-hesitancy-remain-complex
3)
Haelle, T. (2016, December 02). Influences and beliefs on vaccine hesitancy remain complex. Retrieved January 4, 2017, from http://www.mdedge.com/pediatricnews/article/119345/vaccines/influences-and- beliefs-vaccine-hesitancy-remain-complex
4)
Zerbo, P. O. (2017, January 02). Influenza Infection and Vaccination During Pregnancy and Risk of Autism. Retrieved January 05, 2017, from http://jamanetwork.com/journals/jamapediatrics/article- abstract/2587559
5)
Bryant, K. K. (2016, August 25). Summer flu? Think variant swine influenza virus infection. Retrieved January 05, 2017, from http://www.mdedge.com/pediatricnews/article/111964/infectious-diseases/summer- flu-think-variant-swine-influenza-virus
6)
Lancaster University. (2016, September 30). Universal flu vaccine designed by scientists. ScienceDaily. Retrieved January 29, 2017 from www.sciencedaily.com/releases/2016/09/160930085814.htm
7)
Grohskopf, L. A., Sokolow, L. Z., Broder, K. R., Olsen, S. J., Karron, R. A., & Jernigan, D. B. (2016, August 26). Prevention and Control of Seasonal Influenza with Vaccines. Retrieved January 04, 2017, from http://www.aafp.org/patient-care/public-health/immunizations/influenza.html
8)
AAP COMMITTEE ON INFECTIOUS DISEASES. Recommendations for Prevention and Control of Influenza in Children, 2016-2017. Pediatrics. 2016;138(4):e20162527
9)
Estimating Seasonal Influenza-Associated Deaths in the United States. (2016, December 09). Retrieved January 05, 2017, from https://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm
10)
Influenza. (2016, October 06). Retrieved January 4, 2017, from https://www.cdc.gov/nchs/fastats/flu.htm
11)
CDC. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010;59:1057–62. PubMed
12)
CDCet al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59(No. RR-8):1–62. PubMed
13)
CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013;62(No. RR-7).
14)
Chen R, Holmes EC. The evolutionary dynamics of human influenza B virus. J Mol Evol 2008;66:655–63.
15)
O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004;113:585–93.
16)
CIORAP News – February 21, 2018.