
Alicia Greene, DO, Milton S. Hershey Medical Center, Hershey, PA
Nicholas Pennings, DO, FACOFP, Campbell University School of Osteopathic Medicine, Buies Creek, NC
This paper was submitted as part of Dr. Pennings's application for ACOFP Fellowship, which recognizes exceptional national, state, and local service through teaching, authorship, research, or professional leadership. Visit the ACOFP Fellows page to learn more about fellowship and the nomination process.
ABSTRACT
Objective: The metabolic syndrome is a collection of conditions of risk factors associated with insulin resistance and an increased risk of developing cardiovascular disease (CVD) and type 2 diabetes (T2D). While lifestyle modification and weight loss are common recommendations for metabolic syndrome, there are few reports on the impact of dietary interventions on the components of metabolic syndrome. This study examines the impact of a low calorie, high protein diet combined with individualized coaching on a participant’s criteria for metabolic syndrome.
Methods: 95 subjects over age 18 were longitudinally enrolled between 2014 and 2017. A retrospective chart review was conducted with data points collected at initial enrollment and at 3- month follow up visits from electronic medical records.
Results: We found the percentage of participants meeting the criteria for metabolic syndrome declined 66.3% to 34.7% (p < 0.001). There was statistically significant weight loss of 24.45 lbs. (p < 0.001) and improvement on all five parameters of metabolic syndrome following nutritional intervention. A 50% reduction in insulin resistance was also recognized.
Conclusions: Participants engaged in a low calorie, high protein diet, with coaching support, for 3 months showed improvement in all 5 components of metabolic syndrome and insulin resistance. This study provides further evidence for the benefits of dietary intervention on the treatment of insulin resistance and metabolic syndrome.
INTRODUCTION
The metabolic syndrome is a collection of conditions of risk factors associated with an increased risk of developing coronary heart disease - the leading cause of death in the United States for the past 80 years.1 Insulin resistance is identified as a key component of metabolic syndrome as well as a risk factor for cardiovascular disease (CVD) and type 2 diabetes (T2D). 2,3 Obesity, especially when associated with central adiposity, is also associated with metabolic syndrome.4
Since Reaven first described insulin resistance and metabolic syndrome in 1988,5 numerous definitions of metabolic syndrome have been advanced. The NCEP/ATP III criteria is commonly utilized and includes blood pressure, fasting glucose, triglyceride, high-density lipoprotein (HDL) cholesterol, and waist circumference to identify metabolic syndrome.6 The most recent criteria refined the components to account for medication use and racial differences in waist circumference thresholds for metabolic risk (Table 1). 7
| Table 1. Criteria used for metabolic syndrome6 | ||||
| NCEP/ATP III Guidelines | ||||
| Blood Pressure | SBP ≥130 | and/or | DBP≥ 85 mmHg | |
| Glucose | ≥100 mg/dL | |||
| Triglyceride | ≥150 mg/dL | |||
| Men | Women | |||
| HDL Cholesterol | 40 mg/dL | 50 mg/dL | ||
| Waist Circumference | 40in. (102cm) |
| ||
Each component of metabolic syndrome is associated with health risks. Elevated BP increases the risk for serious cardiovascular complications such as: angina, myocardial infarction (MI), heart failure (HF), stroke, peripheral arterial disease, and abdominal aortic aneurysm (AAA).8 Weight loss is the first-line treatment for hypertension. Lifestyle modification, such as diet and exercise, is recommended for all patients, including those on pharmacologic therapies.8
Waist circumference is a reflection of visceral adiposity, metabolic disease risk and mortality.9,10 In patients with obesity, significant reductions in waist circumference is associated with improvement in components of metabolic syndrome.11
Atherogenic dyslipidemia refers to elevated levels of triglycerides and small-dense low- density lipoprotein, as well as low levels of high-density lipoprotein cholesterol. 12 Plasma triglyceride and HDL cholesterol levels are independently associated with insulin resistance and are independent predictors of cardiovascular disease. 13
Hyperglycemia in the prediabetic or diabetic range is associated with increased risk of CVD. 14 While the benefits of improving glycemic control with intensive lifestyle intervention in CVD is controversial, 15 lifestyle interventions improve glycemic control in T2D and, in some cases, can result in remission. 16 Hyperglycemia is typically a later consequence of metabolic syndrome since it normally results from insulin resistance.
Insulin resistance is defined as resistance to insulin stimulated glucose uptake in target cells such as adipose, muscle, and liver cells, resulting in increased circulating glucose levels.17
Variations in glucose uptake are thought to be due to adiposity, fitness, and genetic origin.18 Hyperinsulinemia and/or insulin resistance appears to be a syndrome that is associated with an array of metabolic and physiologic disorders, including weight gain, obesity, non-insulin- dependent diabetes mellitus, hypertension, lipid abnormalities, and atherosclerotic cardiovascular disease. 20-22 Studies reveal that weight loss has a significant effect on the degree of improvement in insulin sensitivity in patients with obesity who participated in low calorie, low carbohydrate, high protein diets. 23,24
To this date, there have been limited studies investigating the effect of specific dietary interventions on components of metabolic syndrome, insulin levels or insulin resistance. Our goal is to analyze the impact of a reduced calorie, high protein diet on parameters of metabolic syndrome and insulin resistance in patients with an overweight BMI or obesity.
MATERIALS AND METHODS
This is a longitudinal study using data from patients during the initial three months on a low-calorie meal replacement plan (Optavia, Baltimore MD) combined with individualized coaching at a single University clinic site located in a rural and underserved county. Dietary intervention consisted of 5 meal replacements consumed approximately every 3 hours, plus one self-prepared, lean protein and vegetable meal. Total calorie count was 800 to 1000 calories per day, with meals composed of approximately 40% protein, 40% carbohydrate, and 20% fat.
Individualized coaching included regular contact by phone or text. Daily contact was made for the first 5 days of the program, then weekly for the remainder of the 3-month trial. Patients also had monthly office encounters with a health care provider. Data was used for any appointment made +/- 1 month of this target. Data points were collected by review of the electronic medical record at initial enrollment in the program, and at follow-up visits scheduled 3 months from the original start date.
Patients were longitudinally enrolled between the years of 2014 and 2017. All participants in this study were 18 years of age or older. Patients were excluded if they had no follow-up appointment data found in the electronic medical record chart review. If patients had partial data, it was included in final statistical analysis using pairwise exclusion for each variable missing. A total of 94 subjects were included overall in this study.
Statistical Analysis
Frequencies and percentages were used to describe categorical variables, while means and standard deviations were used to describe continuous variables. Paired samples t-tests were used to examine the difference in mean values between the continuous variables within the same patient. Pairwise exclusion was utilized to preserve the data we collected during a follow-up visit, while accounting for missing blood draw and laboratory information. All analyses were performed using IBM SPSS Statistics for Macintosh, Version 21.0 (IBM Corp., Armonk, NY). All analyses were deemed significant at α = 0.05.
Weight Change (lbs.)
Participant weight was measured on a Tanita SC-331S body composition analyzer at both the initial visit and 3-month follow-up. The difference between these two weights was used to generate our weight change variable.
HOMA-IR
Insulin resistance was calculated from fasting insulin and fasting glucose (Labcorp, Burlington, NC) values collected at both the initial visit and 3-month follow-up visits using the following formula:
Insulin Resistance (HOMA-IR) = [Fasting Insulin (µU/mL) * Fasting Glucose (mg/dL)] / 405.25
Metabolic Syndrome
For the purposes of this study, the criteria established by a 2009 published joint statement has been used to diagnose metabolic syndrome.7 A patient was considered to have the criteria for metabolic syndrome if >3 of the following were met: elevated waist circumference (using AHA/NHLBI guidelines of > 102cm for men, > 88cm for women), 6 elevated triglycerides (>150mg/dL, or currently treated with medication), low HDL-C (<40mmol/L in men, <50mmol/L in women, or currently treated with medication), hypertension (as defined by systolic blood pressure > 130, diastolic blood pressure > 85, or currently treated with medication), and elevated fasting glucose (>100mg/dL, or currently treated with medication).
Covariates
Age, BMI, gender, and race were evaluated during this study. BMI was categorized as either normal (< 25 kg/m2), overweight (25-29.99 kg/m2), or obese (> 30 kg/m2). The study was IRB approved through the Institutional Review Board of Campbell University.
RESULTS
On average, the study population was 50.1 years of age (20-81, SD=12.88), with a 33.7% male and 66.3% female gender distribution. Race distribution was 88.4% non-Hispanic white, 1.1% non-Hispanic black, and 10.5% unreported. Patients with obesity made up 84.2% of the patient population, overweight 13.7%, and normal 1.1% (Table 2).
Table 2. Study population demographics and metrics.
| Characteristic | 0 Months (n = 94) | 3 Months (n = 94) | p-value |
| Weight (lbs) | 236.33 (64.37) | 211.88 (59.89) | <0.001* |
| BMI (kg/m2) | 37.75 (8.87) | 33.66 (8.11) | <0.001* |
| Body Fat Percentage (%) | 43.32 (8.32) | 39.04 (9.60) | <0.001* |
| Systolic BP (mmHg) | 128.26 (12.63) | 123.39 (12.74) | 0.002* |
| Diastolic BP (mmHg) | 79.47 (7.98) | 74.36 (8.49) | <0.001* |
| Fasting Blood Glucose (mg/dL) | 121.03 (44.70) | 106.21 (32.80) | 0.002* |
| Insulin (mIU/L) | 27.10 (14.99) | 15.76 (11.32) | <0.001* |
| Insulin Resistance (HOMA2-IR) | 8.03 (5.08) | 4.03 (3.29) | <0.001* |
| Hemoglobin A1C (%) | 6.63 (1.12) | 5.86 (0.60) | <0.001* |
| Waist Circumference (in) | 47.20 (5.83) | 43.24 (5.70) | <0.001* |
| Total Cholesterol (mg/dL) | 184.96 (36.45) | 164.16 (30.39) | <0.001* |
| Triglycerides (mg/dL) | 154.22 (75.65) | 110.38 (48.66) | <0.001* |
| HDL (mg/dL) | 45.24 (13.61) | 47.07 (12.69) | 0.172 |
| LDL (mg/dL) | 109.59 (31.32) | 95.30 (26.46) | <0.001* |
*indicates statistically significant result at p<0.005.
Effect of Weight Loss on the Components and Diagnosis of Metabolic Syndrome (Table 3)
Table 3. Impact of weight loss intervention on participants meeting criteria for metabolic syndrome.
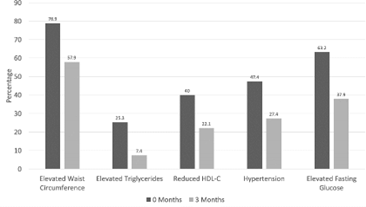
| Diagnostic Component | 0 Months (n = 94) | 3 Months (n = 94) | p-value | |
| Elevated Waist Circumference (%) | 78.9 | 57.9 | < 0.001* | |
| Elevated Triglycerides (%) | 25.3 | 7.4 | < 0.001* | |
| 40.0 | 22.1 | < 0.001* | |
| Hypertension (%) | 47.4 | 27.4 | 0.003* | |
| Elevated Fasting Glucose (%) | 63.2 | 37.9 | < 0.001* | |
| Metabolic Syndrome Diagnosis (%) | 66.3 | 34.7 | < 0.001* |
*indicates statistically significant result at p<0.005.
Figure 1
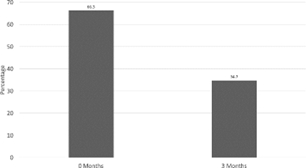
66.3% of patients met criteria for the diagnosis of metabolic syndrome at the start of the study, while only 34.7% met criteria at the end of the study (t = 6.587, df = 94, p < 0.001) (Figure 1). At the beginning of the trial, the average weight of the participants was 236.33lbs. After the 3-month intervention, the average weight decreased to 211.88lbs (p < 0.001). 47.4% of patients were considered hypertensive, with 42.1% on at least one antihypertensive medication. After the intervention, only 27.4% were considered to be hypertensive (t = 3.019, df = 94, p = 0.003), with 33.7% still on at least one antihypertensive medication (t = 2.940, df = 94, p = 0.004). 63.2% of patients were considered hyperglycemic, with 17.9% on at least one medication for glucose control. After the intervention, only 37.9% were considered hyperglycemic (t = 4.688, df = 94, p < 0.001), with no significant decrease in medication use.
25.3% of patients were considered to have high triglycerides, with 5.3% on medication to treat it. After the intervention, only 7.4% of patients were considered to have hypertriglyceridemia (t = 4.233, df = 94, p < 0.001) with no significant reduction in medication use. There was no significant increase in HDL levels after intervention. There was however a statistically significant decrease in the number of participants meeting the standards for a low HDL level according to the metabolic syndrome criteria. 40% were considered to have a low HDL, with no patient on HDL increasing medications. After the intervention, 22.1% had a low HDL (t = 3.609, df = 94, p < 0.001) with no change in HDL increasing medications. 78.9% were considered to have an elevated waist circumference initially, with only 57.9% after the intervention (t = 3.918, df = 94, p < 0.001)
(Figure 2).
Effect of Weight Loss on Insulin Resistance
Overall, there was a statistically significant decline in fasting blood sugar in the study population (t = 3.191, df = 66, p = 0.002). On average, patients began with a fasting blood glucose of 121.03 (SD = 44.70) and ended three months later with an average of 106.21 (SD = 32.80). Fasting insulin levels also saw a statistically significant decline (t = 5.813, df = 50, p < 0.001). On average, patients began with an insulin level of 27.1 (SD = 15.0) and ended with a level of 15.8 (SD = 11.3). Insulin resistance was calculated using HOMA-IR (described above). A statistically significant decline in insulin resistance was seen (t = 6.224, df = 50, p < 0.001).
On average, patients began with HOMA-IR values of 8.03 (SD = 5.08) and ended with values of 4.03 (SD = 3.29).
DISCUSSION
The metabolic syndrome is becoming common worldwide and is highly associated with an increased risk of developing atherosclerotic cardiovascular disease (ASCVD) and type 2 diabetes (T2D).26 According to one NHANES study, it was estimated that the prevalence of metabolic syndrome in U.S. adults in 2011-2016 was 34.7%, affecting 35.1% of women and 34.3% of men. 27 This was a significant increase from 1988-1994 where the prevalence was 28.4% in women and 16.8% in men. 28 Early identification of the parameters of metabolic syndrome in the clinical setting and intensive lifestyle modifications for these individuals has become increasingly important.
In previous studies and reviews, there has been discrepancy identifying the pathophysiology of the metabolic syndrome, which has made it difficult to prevent or improve the syndrome with one technique. Historically, the typical clinical management of metabolic syndrome was to treat each component of the disease separately. 29 Pharmaceutical therapies are targeted to lower blood pressure, blood glucose, and triglycerides, as well as address the prothrombic state, which produces a piecemeal treatment approach without addressing the underlying cause. 30
Lifestyle intervention through dietary restriction, increased physical activity, and behavioral modification is frequently touted as first-line therapy for the treatment of metabolic syndrome. 31-33 Much of the support for lifestyle intervention has centered around individual components with limited data on the impact of intensive lifestyle or nutritional intervention on metabolic syndrome as a whole. 30 Root analyzed the OMNI Heart dataset examining 4 of the 5 components of metabolic syndrome utilizing a DASH-like diet with weight-neutral effects. The study found overall improvement in metabolic syndrome based on the measured components, absent waist circumference, especially for participants on a high protein, high fat diet. 34
This study is unique in that it examined the impact of lifestyle modification with resultant weight loss on not just the individual parameters of metabolic syndrome but the number of participants meeting criteria for metabolic syndrome. The percentage of participants meeting at least 3 of the 5 components of metabolic syndrome declined 66.3% to 34.7%, or a 48% reduction in participants meeting criteria for metabolic syndrome. There was a statistically significant average weight loss of 24.45lbs per participant during the 3-month intervention (p<0.001).
Metabolic improvements from normalizing components of metabolic syndrome may benefit individuals by decreasing their risk for developing CVD and diabetes.
We also found statistically significant improvement (p<0.001 elevated waist circumference, elevated triglycerides, elevated fasting glucose, reduced HDL-C; p=0.003 elevated blood pressure) in the percentage of participants meeting criteria for each individual parameter of metabolic syndrome following nutritional intervention.
In addition to a reduction in metabolic syndrome, a 50% reduction in insulin resistance was also recognized in this study. Metabolic syndrome is associated with impaired insulin- dependent vasodilatation, and lifestyle intervention has been shown to reverse vascular dysfunction, with potential improvement in vascular consequences associated with CVD and diabetes. 35
Generalizability of this study may be limited due to our patient population. This study included 66.3% women, and overall 88.4% Caucasian individuals. Different populations, ethnicities, and nationalities have different distributions of norms for body weight and waist circumference. 29 In this particular study, though each patient received a similar diet plan and individualized coaching, individual exercise routines were not quantified during the study.
Regular exercise plays an important role in abdominal fat loss and the prevention of fat regain in those who have successfully lost weight. 29 The time period of this study was limited to 3 months. Additional study is needed to evaluate the longitudinal impact on the sustainability of improvements in metabolic syndrome as well as the impact on CVD and T2D.
CONCLUSION
This study showed that a high protein, low calorie diet resulted in weight loss and significantly reduced the number of participants meeting criteria for metabolic syndrome. Among individuals meeting criteria for metabolic syndrome, 48% no longer met criteria at the end of the 3-month intervention. Statistically significant improvements were also noted for all individual components of metabolic syndrome and insulin resistance. This demonstrates potential targets for interventions to improve metabolic syndrome. Further study is needed to assess long-term impact on metabolic syndrome, CVD and T2D.
Acknowledgments: The authors thank Amy Allen, RN, CDE, for her contribution in participant coaching and data collection, as well as Peter Ahiawodzi, PhD, for his assistance with data analysis.
Funding: No grants, equipment, drugs, or reagents were used in this trial.
Author Contributions: AG participated in data collection, data analysis, and document writing. NP participated in research design, data collection, data analysis, and document writing. All authors read and approved the final manuscript.
Conflict of Interest Statement: Dr. Nicholas Pennings has an independent contractor relationship with Medifast, Inc., is a consultant with the Obesity Medicine Association and an educational consultant for Novo Nordisk, Gelesis, and Eli Lilly. Dr. Alicia Greene declares no conflict of interest.
REFERENCES
- Ward JW, Warren C. Silent Victories: The History and Practice of Public Health in Twentieth-Century America. Oxford University Press; 2006.
- Johnson, J. D. “On the causal relationships between hyperinsulinaemia, insulin resistance, obesity and dysglycaemia in type 2 diabetes.” Diabetologia, 64.10 (2021): 2138-2146.
- Ormazabal, V., Nair, S., Elfeky, O., Aguayo, C., Salomon, C., & Zuñiga, F. A. “Association between insulin resistance and the development of cardiovascular disease.” Cardiovascular diabetology, 17.1(2018): 1-14.
- O’Neill S, O’Driscoll L. “Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies.” Obes Rev Off J Int Assoc Study Obes. 16.1(2015):1–12.
- Reaven GM. “Banting lecture 1988. Role of insulin resistance in human disease.” Diabetes, 37.12(1988): 1595–1607.
- Wilkins LW. et al. “Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report.” Circulation, 106.25(2002): 3143–3143.
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman, JI, Donato KA, … Smith S. 2009;120.16(2009): 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644Circulation C. “Harmonizing the Metabolic Syndrome.”
- Whelton PK., Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, … Wright JT. “2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA
Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.” Hypertension (Dallas, Tex.: 1979), 71.6(2018): e13–e115. https://doi.org/10.1161/HYP.0000000000000065
- Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, … Berrington de Gonzalez A. “A pooled analysis of waist circumference and mortality in 650,000 adults.” Mayo Clinic Proceedings, 89.3(2014): 335–345. https://doi.org/10.1016/j.mayocp.2013.11.011
- Swainson MG, Batterham AM, Tsakirides C, Rutherford ZH, Hind, K. “Prediction of whole- body fat percentage and visceral adipose tissue mass from five anthropometric variables.” PloS One, 12.5(2017), e0177175. https://doi.org/10.1371/journal.pone.0177175
- Rothberg AE, McEwen LN, Kraftson AT, Ajluni N, Fowler CE, Nay CK, … Herman WH. “Impact of weight loss on waist circumference and the components of the metabolic syndrome.” BMJ Open Diabetes Research & Care, 5.1(2017): e000341. https://doi.org/10.1136/bmjdrc-2016-000341
- Manjunath CN, Rawal JR, Irani PM, Madhu K. “Atherogenic dyslipidemia.” Indian Journal of Endocrinology and Metabolism, 17.6(2013): 969–976. https://doi.org/10.4103/2230- 8210.122600
- Lechner, K., McKenzie, A. L., Kränkel, N., Von Schacky, C., Worm, N., Nixdorff, U., ... & Krauss, R. M. “High-risk atherosclerosis and metabolic phenotype: the roles of ectopic
adiposity, atherogenic dyslipidemia, and inflammation.” Metabolic syndrome and related disorders, 18.4(2020): 176-185.
- Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, & Manson JE. “Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes.” Diabetes Care, 25.7(2002): 1129–1134.
- Gerstein HC. “Do lifestyle changes reduce serious outcomes in diabetes?” The New England Journal of Medicine, 369.2(2013): 189–190. https://doi.org/10.1056/NEJMe1306987
- Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, … “Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes.” JAMA, 308.13(2012): 2489–2496. https://doi.org/10.1001/jama.2012.67929
- Reaven GM. “The insulin resistance syndrome: definition and dietary approaches to treatment.” Annu. Rev. Nutr., 25(2005): 391-406.
- Huang PL. “A comprehensive definition for metabolic syndrome.” Disease Models & Mechanisms, 2.5-6(2009): 231–237. https://doi.org/10.1242/dmm.001180
- Liese AD, Mayer-Davis EJ, Haffner SM. “Development of the multiple metabolic syndrome: an epidemiologic perspective.” Epidemiologic Reviews, 20.2(1998): 157–172.
- DeFronzo RA, Ferrannini E. Insulin resistance. “A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease.” Diabetes Care, 14.3(1991): 173–194.
- Thomas DD, Corkey BE, Istfan NW, Apovian CM. “Hyperinsulinemia: an early indicator of metabolic dysfunction. Journal of the Endocrine Society.” 3.9(2019): 1727-47
- Pennings N, Jaber J, Ahiawodzi P. “Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation.” Diabetes/Metabolism Research and Reviews, 34.4(2018): e2986. https://doi.org/10.1002/dmrr.2986
- Huguenin GVB, Uehara SK, Netto JFN, Gaspar de Moura E, Rosa G, & Passos MCda F. “Short term low-calorie diet improves insulin sensitivity and metabolic parameters in obese women.” Nutricion Hospitalaria, 30.1(2014): 53–59. https://doi.org/10.3305/nh.2014.30.1.7464
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Stern L. “A Low- Carbohydrate as Compared with a Low-Fat Diet in Severe Obesity.” New England Journal of Medicine, 348.21(2003): 2074–2081. https://doi.org/10.1056/NEJMoa022637
- Wallace TM, Levy JC, & Matthews DR. “Use and abuse of HOMA modeling.” Diabetes Care, 27.6(2004): 1487–1495.
- Isomaa B, Almgren P, Tuomi T, et all. “Cardiovascular morbidity and mortality associated with the metabolic syndrome.” Diabetes Care. 24.4(2001): 683-689. doi:10.2337/diacare.24.4.683
- Hirode, Grishma, and Robert J. Wong. "Trends in the prevalence of metabolic syndrome in the United States, 2011-2016." Jama 323.24 (2020): 2526-2528.
- Mozumdar A, Liguori G. “Persistent increase of prevalence of metabolic syndrome among
U.S. adults: NHANES III to NHANES 1999-2006.” Diabetes Care, 34.1(2011): 216–219. https://doi.org/10.2337/dc10-0879
- Kaur, Jaspinder. "A comprehensive review on metabolic syndrome." Cardiology research and practice 2014 (2014). https://doi.org/10.1155/2014/943162
- Grundy SM. “Metabolic syndrome update.” Trends in Cardiovascular Medicine, 26.4(2016): 364–373. https://doi.org/10.1016/j.tcm.2015.10.004
- Andersen CJ, Fernandez ML. “Dietary strategies to reduce metabolic syndrome.” Reviews in Endocrine & Metabolic Disorders, 14.3(2013): 241–254. https://doi.org/10.1007/s11154- 013-9251-y
- Mechanick JI, Garber AJ, Grunberger G, Handelsman Y, Garvey WT. “Dysglycemia-based chronic disease: an American Association of Clinical Endocrinologists position statement.” Endocrine Practice. 11(2018): 995-1011. doi.org/10.4158/PS-2018-0139
- Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, … “Endocrine Society. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline.” The Journal of Clinical Endocrinology and Metabolism, 93.10(2008): 3671–3689. https://doi.org/10.1210/jc.2008-0222
- Root MM, Dawson HR. “DASH-like diets high in protein or monounsaturated fats improve metabolic syndrome and calculated vascular risk.” International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift Fur Vitamin- Und Ernahrungsforschung. Journal International De Vitaminologie Et De Nutrition, 83.4(2013): 224–231. https://doi.org/10.1024/0300-9831/a000164
- Vinet A, Obert P, Dutheil F, Diagne L, Chapier R, Lesourd B, Walther G. “Impact of a lifestyle program on vascular insulin resistance in metabolic syndrome subjects: the RESOLVE study.” The Journal of Clinical Endocrinology and Metabolism, 100.2(2015): 442–450. https://doi.org/10.1210/jc.2014-2704





