Abstract
Corresponding Author(s)
Yoshihiro Ozaki, DO, FAAP | yozaki@valleychildrens.org
The authors have no conflicts of interest or financial disclosures.
Read the article
A 14-year-old previously healthy female was admitted to our institution with extensive left foot and ankle cellulitis. She had a history of tinea pedis and multiple skin breakdowns. The patient visited a beach where she walked barefoot. Before admission, she had a 5-day history of scaling and pustules between her toes, which continued to worsen despite treatment with cephalexin and clotrimazole cream. Three days before admission, she developed fever, limping, and worsening erythema and swelling of her left foot and ankle.
Physical examination revealed an ill-appearing and diaphoretic teenage female with swelling in her left foot and ankle. Her temperature was 37.1°C, with a maximum of 38.5°C. Her blood pressure was 94/52 mmHg, heart rate was 112 beats/min, respiratory rate was 16 breaths/min, and oxygen saturation was 98% on room air. Her weight was 53.6 kg at admission. She had good perfusion with a capillary refill time of less than 2 seconds. On musculoskeletal and skin examination, she had erythematous swelling of the dorsal and plantar aspects of her left foot, with swelling of the left ankle and pain on flexion and rotation. Point tenderness on palpation was noted on the medial malleolus. Interdigital skin breakdowns with an erythematous base without pustules or drainage were also noted.
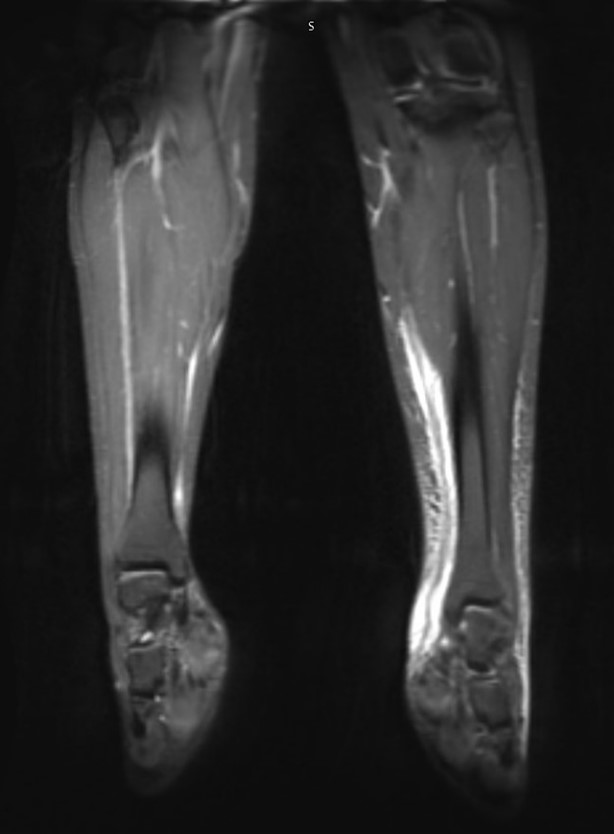
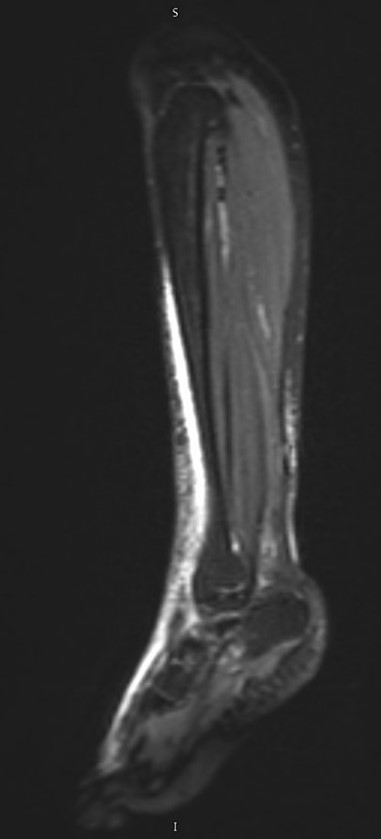
Laboratory testing revealed a complete blood count (CBC) with a white blood cell count of 18,500 cells/µL (90% neutrophils, 4.5% lymphocytes, 4.4% monocytes, 1% eosinophils), hemoglobin of 10.9 g/dL, and a platelet count of 247,000 cells/µL. C-reactive protein (CRP) was 7.6 mg/dL, and procalcitonin was 0.24 ng/mL. The basic metabolic panel (BMP) showed sodium 138 mmol/L, potassium 3.3 mmol/L, chloride 108 mmol/L, bicarbonates 20 mmol/L, blood urea nitrogen (BUN) 8.0 mg/dL, creatinine 0.46 mg/dL, glucose 107 mg/dL, and calcium 8.9 mg/dL. Blood culture was negative. Magnetic resonance imaging (MRI) of the left tibia, foot, and ankle was ordered with concerns of osteomyelitis, septic joint, and necrotizing fasciitis, and showed edema up to the midmedial gastrocnemius level adjacent to fascial structures of the anterior and posterior compartments of the left lower extremity, extending onto the dorsum of the foot (Figure 1).
FIGURE 1. MRI of left tibia, foot, and ankle with contrast
(A) Coronal view and (B) sagittal view MRI images showing edema adjacent to the fascial structures of the anterior and posterior compartments of the left lower extremity, extending onto the dorsum of the foot.
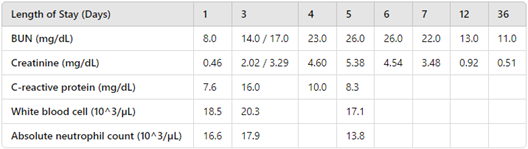
Upon admission, the patient received one dose of piperacillin-tazobactam 3 g (of piperacillin) intravenously (IV) and two doses of vancomycin 1000 mg IV for possible necrotizing fasciitis, as well as one dose of ibuprofen 400 mg. Once the MRI of the left foot and ankle ruled out septic joint, osteomyelitis, and necrotizing fasciitis, antibiotics were switched to clindamycin 720 mg (40 mg/kg/day divided every 8 hours) to treat extensive cellulitis including methicillin-resistant Staphylococcus aureus (MRSA) shortly after admission. On day 3 of hospitalization, the patient reported dark urine. The basic metabolic profile showed BUN 17.0 mg/dL and creatinine 2.02 mg/dL (Table 1). Urinalysis showed protein of 30 mg/dL and moderate blood with red blood cells of 85/µL, with negative pyuria, negative leukocyte esterase, and negative nitrites. While on monotherapy with clindamycin, without nephrotoxic medications such as ibuprofen, renal function continued to worsen with BUN 26.0 mg/dL and creatinine 5.38 mg/dL by day 5 of hospitalization. BUN and creatinine trending are described in Table 1. Inflammatory markers started showing an improvement on day 4. The patient also required amlodipine for hypertension associated with acute kidney injury (AKI). Due to concern for AKI from clindamycin, the antibiotic was switched to linezolid 600 mg IV every 12 hours. Over the next 2 days, BUN and creatinine improved to 22.0 mg/dL and 3.48 mg/dL, respectively. The patient was discharged home with a total 14-day course of linezolid. She was followed by nephrology as an outpatient. BUN and creatinine normalized at 1-month follow-up.
TABLE 1. Laboratory trends of BMP, CRP, and CBC
DISCUSSION
Clindamycin remains one of the commonly used antibiotics for treating skin and soft-tissue infections (SSTIs), particularly in light of the 25-fold increase in hospital admissions of children with MRSA infections in the 1990s.5[AS1] Despite a 52% decline in hospital admissions due to MRSA, the infection continues to pose a significant threat to pediatric populations.6 Clindamycin has good penetration into bone and joint tissues, as well as high bioavailability with oral administration, which contributes to its widespread use in both outpatient and inpatient settings.7 Nephrotoxicity is a common side effect of many medications, but it is rarely caused by clindamycin. We expound the limited clinical evidence for AKI with clindamycin, highlighting that while documented cases in adults exist, pediatric instances remain exceptionally rare, thus underscoring the significance of our case report.
Several mechanisms of medication-induced nephrotoxicity have been identified: alteration of glomerular filtration rate, tubular cell toxicity, interstitial nephritis, and crystal nephropathy.8 Based on biopsy case series, acute interstitial nephritis and acute tubular necrosis were discovered among patients with clindamycin-induced AKI.9 One retrospective analysis of adult patients reported that reversible clindamycin-induced kidney disease occurred on average 1.5 days after the first dose, with patients receiving 1.0 to 2.0 g of clindamycin per day.10 Common manifestations include proteinuria, gross hematuria, oliguria, edema, nausea, vomiting, abdominal pain, and diarrhea.
In this case, the patient initially received nephrotoxic medications including piperacillin-tazobactam, vancomycin, and ibuprofen briefly. Lexicomp reported increased cases of AKI when vancomycin was concurrently used with either ibuprofen or piperacillin. Although one dose of piperacillin-tazobactam and ibuprofen, and two doses of vancomycin, may have contributed to nephrotoxicity in this case, the persistently worsening AKI while on monotherapy with clindamycin for 4 days raises concerns about the nephrotoxic nature of clindamycin. According to Lexicomp, clindamycin did not have any drug interactions with these three medications. After clindamycin was switched to linezolid, serial basic metabolic profiles showed persistent improvement.
CONCLUSION
Our case underscores the importance of considering AKI as a rare but potential side effect of clindamycin among pediatric patients, as clindamycin is commonly prescribed in both outpatient and inpatient pediatrics. This case highlights that a high level of clinical suspicion can help clinicians facilitate appropriate evaluation and treatment for reversible clindamycin-induced AKI.
REFERENCES
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147–159. doi:10.1093/cid/ciu444
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516–1518. doi:10.3201/eid1509.081228
- Clindamycin drug interactions. In: Lexi-Drugs Online. [database online]. Wolters Kluwer Health, Inc.; 2024. Accessed May 26, 2024. https://online.lexi.com
- Subedi P, Chowdhury A, Tanovic K, Dumic I. Clindamycin: an unusual cause of acute kidney injury. Am J Case Rep. 2019;20:248–251. doi:10.12659/AJCR.913779
- Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279(8):593–598. doi:10.1001/jama.279.8.593
- Spaulding AB, Thurm C, Courter JD, et al. Epidemiology of Staphylococcus aureus infections in patients admitted to freestanding pediatric hospitals, 2009-2016. Infect Control Hosp Epidemiol. 2018;39(12):1487–1490. doi:10.1017/ice.2018.259
- Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–136. doi:10.1016/j.ijid.2019.02.005
- Patel JB, Sapra A. Nephrotoxic Medications. In: StatPearls [Internet]. 2023. Accessed May 26, 2024. http://www.ncbi.nlm.nih.gov/books/NBK532864/
- Xie H, Chen H, Hu Y, et al. Clindamycin-induced acute kidney injury: large biopsy case series. Am J Nephrol. 2013;38(3):179–183. doi:10.1159/000354088
- Wan H, Hu Z, Wang J, Zhang S, Yang X, Peng T. Clindamycin-induced kidney diseases: a retrospective analysis of 50 patients. Intern Med. 2016;55(11):1433–1437. doi:10.2169/internalmedicine.55.6084